Magnetite is a very common iron oxide (Fe3O4) mineral that is found in igneous, metamorphic, and sedimentary rocks. It is the most commonly mined ore of iron. It is also the mineral with the highest iron content (72.4%). An oversimplified synthesis reaction (Figure 1) demonstrates the chemical makeup of the compound [1], [2].
Cubic inverse spinel magnetite (Fe3O4) is one of the most researched magnetic materials. This oxide is widely used in magnetic biomedicine, heavy metal ions removal, electromagnetic wave absorption and other fields. This growing interest of Fe3O4 is due to its unique characteristic such as strong magnetism, long durability, good biocompatibility, low toxicity and low cost [3], [4].
Structural
Magnetite’s crystal structure follows an inverse spinel pattern with alternating octahedral and tetrahedral-octahedral layers. From Figure 2 (b), ferrous species are observed to occupy half of the octahedral lattice sites due to greater ferrous crystal field stabilization energy (CFSE); alternatively, ferric species occupy the other octahedral lattice sites and all tetrahedral lattice sites and oxygen forms an fcc closed-pack structure [5], [6].Figure 3 shows the XRD peaks of α-Fe2O3, Fe3O4 and γ-Fe2O3 [7].
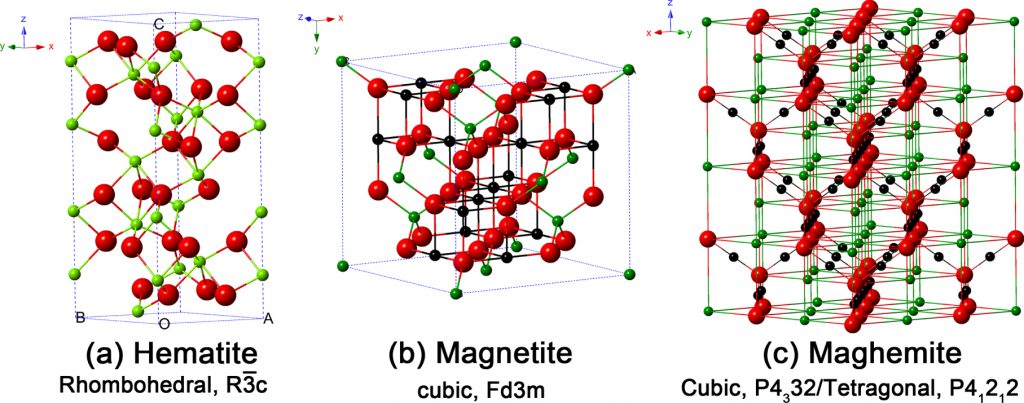 Figure 1 Synthesis reaction of iron oxide [1].
Figure 1 Synthesis reaction of iron oxide [1].
 Figure 2 Crystal structure, crystallographic data and particle’s color of the hematite, magnetite and maghemite (the black ball is Fe2+, the green ball is Fe3+ and the red ball is O2−) [۷], [۸].
Figure 2 Crystal structure, crystallographic data and particle’s color of the hematite, magnetite and maghemite (the black ball is Fe2+, the green ball is Fe3+ and the red ball is O2−) [۷], [۸].
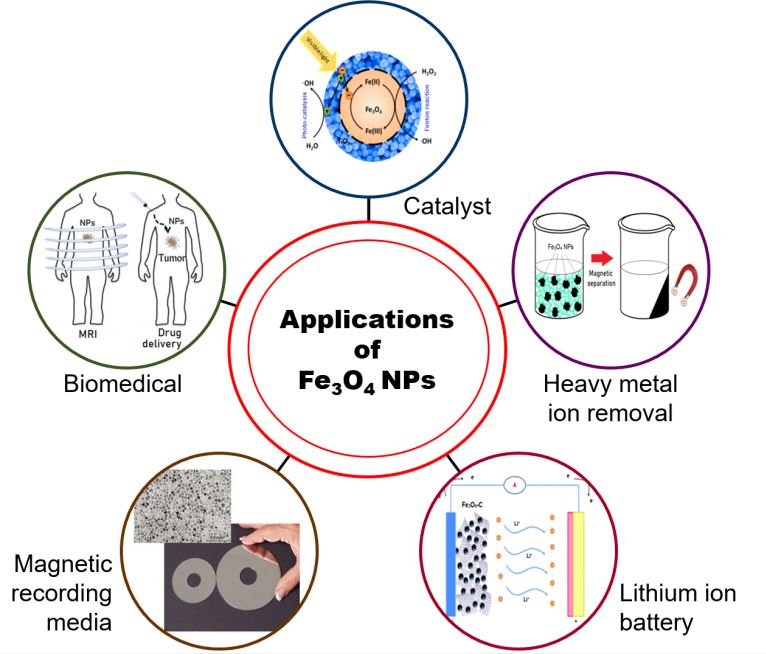 Applications of magnetite nanoparticles (Fe3O4-NPs)
Applications of magnetite nanoparticles (Fe3O4-NPs)
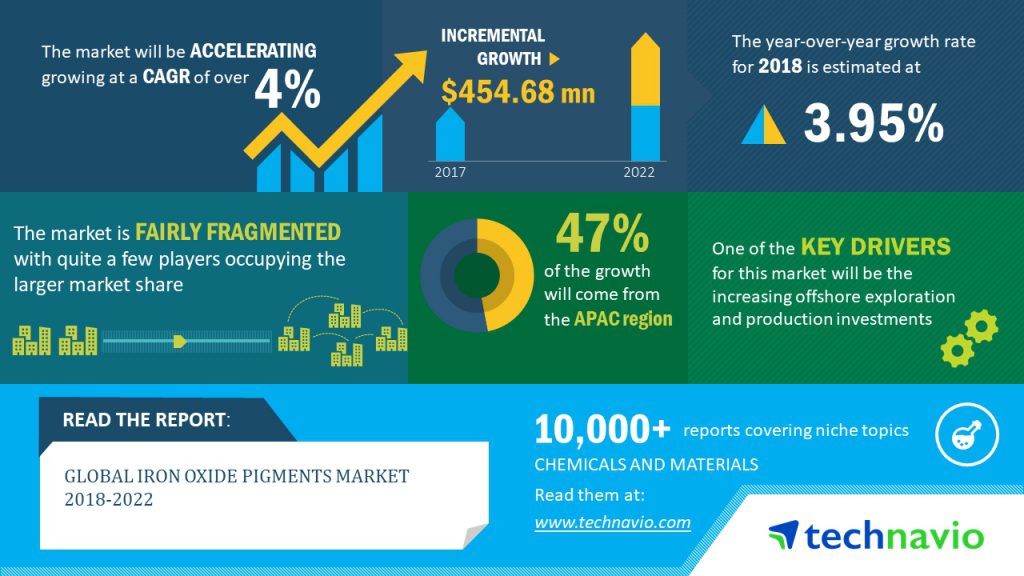 Global iron oxide pigments market
Global iron oxide pigments market
References:
[۱] L. Blaney, “Magnetite (Fe3O4): Properties, Synthesis, and Applications,” Lehigh Rev., vol. 15, no. 5, pp. 33–۸۱, ۲۰۰۷٫
[۲] “Magnetite and Lodestone.” [Online]. Available: https://geology.com/minerals/magnetite.shtml.
[۳] L. H. Reddy, J. L. Arias, J. Nicolas, and P. Couvreur, “Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications,” Chem. Rev., vol. 112, no. 11, pp. 5818–۵۸۷۸, ۲۰۱۲٫
[۴] M. Colombo et al., “Biological applications of magnetic nanoparticles,” Chem. Soc. Rev., vol. 41, no. 11, pp. 4306–۴۳۳۴, ۲۰۱۲٫
[۵] Y. Tao, X.-D. Wen, R. E. N. Jun, Y.-W. Li, J.-G. Wang, and C.-F. Huo, “Surface structures of Fe3O4 (111),(110), and (001): A density functional theory study,” J. Fuel Chem. Technol., vol. 38, no. 1, pp. 121–۱۲۸, ۲۰۱۰٫
[۶] A. K. Singh, O. N. Srivastava, and K. Singh, “Shape and Size-Dependent Magnetic Properties of Fe3O4 Nanoparticles Synthesized Using Piperidine,” Nanoscale Res. Lett., vol. 12, pp. 0–۶, ۲۰۱۷٫
[۷] W. Wu, Z. Wu, T. Yu, C. Jiang, and W. S. Kim, “Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications,” Sci. Technol. Adv. Mater., vol. 16, no. 2, 2015.
[۸] R. M. Cornell and U. Schwertmann, The iron oxides: structure, properties, reactions, occurrences and uses. John Wiley & Sons, 2003.
