Copper (chem. symbol Cu) as a red-brown metal, is one of the world’s most important industrial metals. Copper is typically extracted from oxide and sulfide ores that contain between 0.5 and 2.0 percent copper. All sulfide-type copper ores including chalcocite (Cu2S), chalcopyrite (CuFeS2) and covellite (CuS), are treated by smelting [1].
Properties
Copper has applications in electrical, catalytic, optical, biomedical, and antimicrobial applications, due to which it is one of the most commonly used materials. Properties of copper nanoparticles can be easily altered by varying their sizes, shapes, and chemical environment.
Electrical and thermal conductivity
Copper has the best electrical conductivity of any metal, except silver. Copper is made up of closely packed atoms with face center cubic structure. Each copper atom has lost one electron and become a positive ion so the ions vibrate and free electrons move between them. Many printed electronics, displays, and conductive thin films use conductive inks and pastes containing noble metals that are very expensive.
Corrosion resistant
Copper is low in the reactivity series. This means that it doesn’t tend to corrode.
Antibacterial
Copper is a naturally hygienic metal that slows down the growth of germs such as E-coli (the “burger bug”), MRSA (the hospital “superbug”) and legionella. This is important for applications such as food preparation, hospitals, coins, door knobs and plumbing systems.
Catalytic compounds
Copper can act as a catalyst. For example, it speeds up the reaction between zinc and dilute sulphuric acid. It is found in some enzymes, one of which is involved in respiration [5].
Applications
The key applications of copper nanoparticles are listed below [6]:
- Acts as an anti-biotic, anti-microbial, and anti-fungal agent when added to plastics, coatings, and textiles;
Nano-copper was reported to be highly effective in controlling bacterial diseases viz. bacterial blight of rice (Xanthomonas oryzae pv.oryzae) and leaf spot of mung (X. campestris pv. Phaseoli) [7].
Copper diet supplements with efficient delivery characteristics
High strength metals and alloys
EMI shielding
Heat sinks and highly thermal conductive materials
Efficient catalyst for chemical reactions and for the synthesis of methanol and glycol;
Copper nanoparticles with great catalytic activities can be applied to biosensors and electrochemical sensors. Redox reactions utilized in those sensors are generally irreversible and also require high overpotentials (more energy) to run. In fact, the nanoparticles have the ability to make the redox reactions reversible and to lower the overpotentials when applied to the sensors. One of the examples is a glucose sensor. Depending on the level of glucose, the nanoparticles in the sensor diffract the incident light at a different angle [8], [9].
As sintering additives and capacitor materials
Conductive inks and pastes containing Cu nanoparticles can be used as a substitute for very expensive noble metals used in printed electronics, displays, and transmissive conductive thin film applications
Superficial conductive coating processing of metal and non-ferrous metal
Production of MLCC internal electrode and other electronic components in electronic slurry for the miniaturization of microelectronic devices;
As nanometal lubricant additives
Market
In terms of market share, North America is leading the global copper nanoparticles market. This trend is likely to continue in the near future. Abundance of raw materials is one of the factor contributing to the dominant share of the market in North America. In terms of growth potential, the market in Asia Pacific is anticipated to lead the market in the next few years. Key players in the copper nanoparticles market include TEKNA, Strem Chemicals Inc., Nano Technology Inc., IoLiTec Ionic Liquids Technologies GmbH, Meliorum Technologies Inc., QuantumSphere Inc., and EPRUI Nanoparticles & Microspheres Co. Ltd [15].
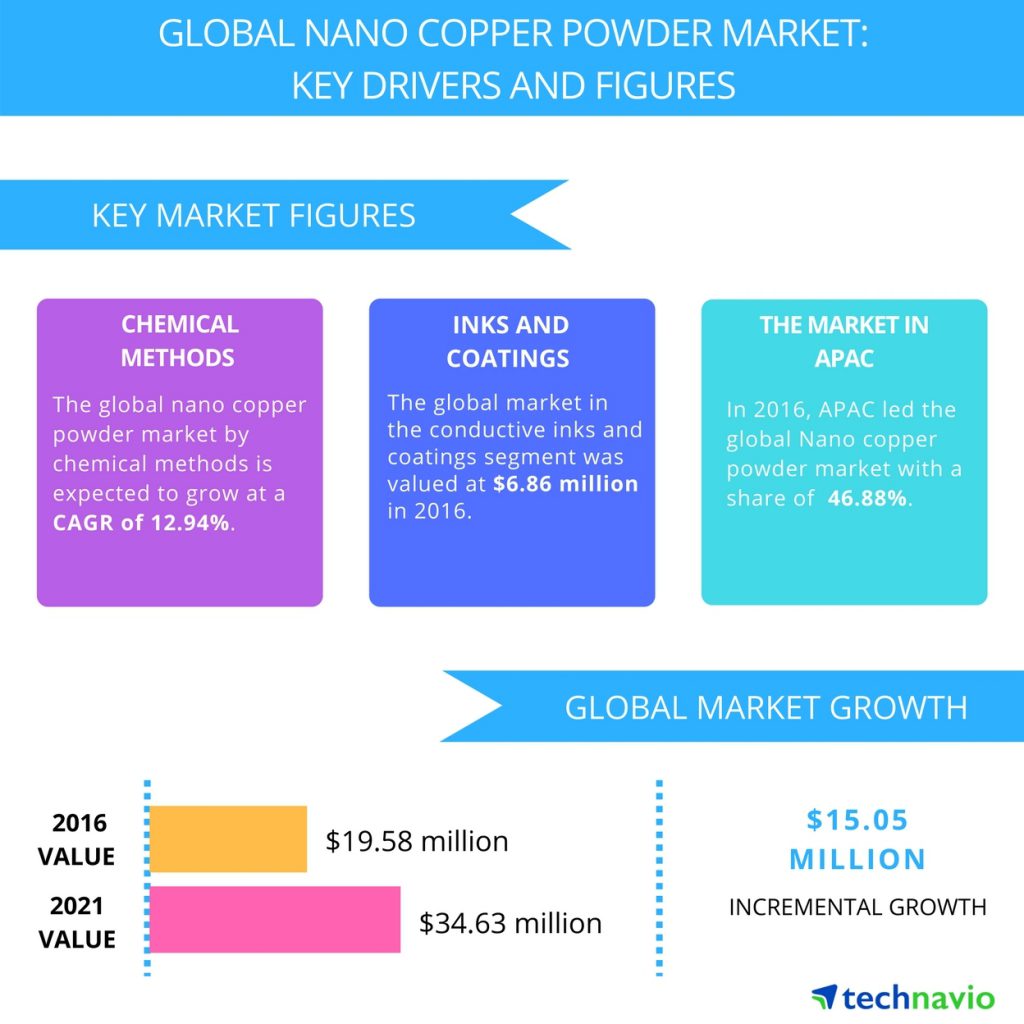
Global nano copper powder market is expected to reach USD 34.63 million by 2021, growing at a CAGR of over 12% (Figure 1). Conductive inks and lubricant additives are the early starters to incorporate and commercialize nano copper based products. The top three emerging trends driving the global nano copper powder market according to Technavio research analysts are:
Nano copper for batteries and capacitors
Nano copper is one of the trending materials for electrodes in batteries, capacitors, and supercapacitors. In the battery segment, Li-ion batteries are the breakthrough and are dominating the segment over the past two decades. However, Li-ion batteries are only used in the portable electronics segment, and they have limitation to serve the large-scale energy storage segment.
Copper nanofluids for diverse end-user markets
Nano copper powder based nanofluids have high conductivity and low viscosity in a wide range of applications. Few challenges relating to homogeneous dispersion of copper nanomaterials and base fluids, stability of suspension, agglomeration, and nano copper shape and size selection for attaining the desired properties play a vital role in the performance of nanofluids.
Lead-free soldering materials to replace tin-lead solders
Due to environmental concerns regarding the use of lead, which is inherently toxic and hazardous to human and the environment, the use of tin-lead solders is restricted. To obtain sufficient wetting by lead-free solders, the processing temperature during electronic assembly must, therefore, be raised by 30°C-40°C. This temperature increase reduces the integrity, reliability, and functionality of printed wiring boards, components, and other attachments [16].
The conductive inks and coatings
This segment offers a wide scope for nano copper and is used in a range of products such as RFID, sensors and detectors, displays, photovoltaics, smart cards, touch screens, EMI and radio frequency interference shielding, OLED displays, and lighting. Also, advantages such as excellent electrical conductivity, lesser cost, and established preparation methods and the rising trend of replacing silver-based conductive inks and the miniaturizing of consumer electronics make nano copper powder one of the vital additions in conductive inks and coatings. The conductive inks and coatings segment accounted for the maximum shares of the nano copper powder market during 2016 and it is the key end-user of nano copper with consumption of nearly 35% of the nano copper production [17].
Figure 1 global nano copper powder market from 2017-2021 [16].
Synthesis methods
The preparation of copper nanoparticles is much more difficult in comparison with noble metals due to the possibility of oxidation of copper with air and agglomeration of particles take place because of surface oxidation
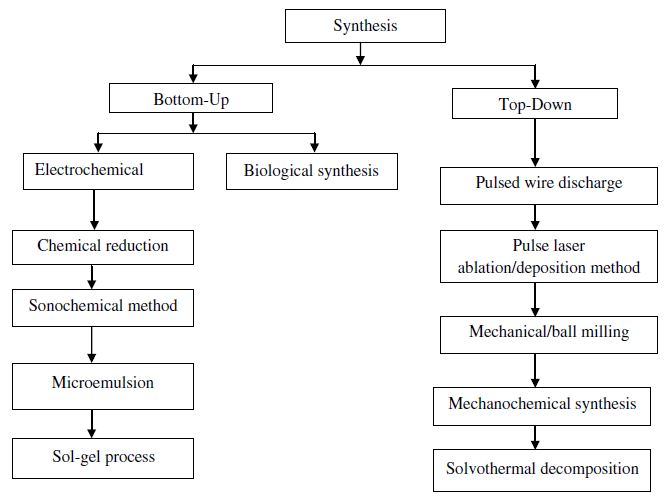 Figure 2 copper nanoparticles preparation methods [19].
Figure 2 copper nanoparticles preparation methods [19].
References:
[۱] “Manufacturing Process of Copper.” [Online]. Available: https://www.thebalance.com/copper-production-2340114.
[۲] “Copper and copper oxides – Overview.” [Online]. Available: https://www.nanopartikel.info/en/nanoinfo/materials/copper-and-copper-oxides/overview.
[۳] “Copper and Copper oxides.” [Online]. Available: https://www.nanopartikel.info/en/nanoinfo/materials/copper-and-copper-oxides/material-information.
[۴] Z. Abdul Majid and Z. Yaacob, “Alternative Piping Material for Malaysian Fuel Gas Distribution,” J. Teknol., vol. 35, no. 1, 2013.
[۵] “copper- a vital element.” [Online]. Available: http://resources.schoolscience.co.uk/CDA/14-16/chemistry/copch0pg5.html.
[۶] “Copper (Cu) Nanoparticles – Properties, Applications.” [Online]. Available: https://www.azonano.com/article.aspx?ArticleID=3271.
[۷] “Nanotechnology in Agriculture.” [Online]. Available: https://www.nanoshel.com/Nanotechnology-in-Agriculture.
[۸] X. Luo, A. Morrin, A. J. Killard, and M. R. Smyth, “Application of Nanoparticles in Electrochemical Sensors and Biosensors,” pp. 319–۳۲۶, ۲۰۰۶٫
[۹] A. K. Yetisen et al., “Reusable, Robust, and Accurate Laser-Generated Photonic Nanosensor,” ۲۰۱۴٫
[۱۰] T. M. D. Dang, T. T. T. Le, E. Fribourg-Blanc, and M. C. Dang, “Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method,” Adv. Nat. Sci. Nanosci. Nanotechnol., vol. 2, no. 1, 2011.
[۱۱] G. Liu, X. Li, B. Qin, D. Xing, Y. Guo, and R. Fan, “Investigation of the mending effect and mechanism of copper nano-particles on a tribologically stressed surface,” Tribol. Lett., vol. 17, no. 4, pp. 961–۹۶۶, ۲۰۰۴٫
[۱۲] P. Zietz, H. H. Dieter, M. Lakomek, H. Schneider, and B. Keßler-, “Epidemiological investigation on chronic copper toxicity to children exposed via the public drinking water supply,” vol. 302, pp. 127–۱۴۴, ۲۰۰۳٫
[۱۳] J. Bertinato and M. R. L’Abbé, “Maintaining copper homeostasis: regulation of copper-trafficking proteins in response to copper deficiency or overload,” J. Nutr. Biochem., vol. 15, no. 6, pp. 316–۳۲۲, ۲۰۰۴٫
[۱۴] M. S. Badanavalu M. Prabhu, Syed F. Ali, Richard C. Murdock, Saber M. Hussain, “Copper nanoparticles exert size and concentration dependent toxicity on somatosensory neurons of rat,” Nanotoxicology, vol. 4, no. 2, pp. 150–۱۶۰, ۲۰۱۰٫
[۱۵] “Copper Nanoparticles Market – Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2017 – 2025.” [Online]. Available: https://www.transparencymarketresearch.com/copper-nanoparticles-market.html.
[۱۶] “Nano Copper Powder Market.” [Online]. Available: https://www.businesswire.com/news/home/20170605005923/en/Nano-Copper-Powder-Market—Trends-Forecasts.
[۱۷] “Global Nano Copper Powder Market 2017-2021.” [Online]. Available: https://www.technavio.com/report/global-nano-copper-powder-market.
[۱۸] A. Tamilvanan and B. Kulendran, “Copper Nanoparticles : Synthetic Strategies , Properties and Multifunctional Application Copper Nanoparticles : Synthetic Strategies , Properties and Multifunctional Application,” no. February 2015, 2014.
[۱۹] R. Bali, N. Razak, A. Lumb, and A. T. Harris, “The synthesis of metallic nanoparticles inside live plants,” in 2006 International Conference on Nanoscience and Nanotechnology, 2006.
[۲۰] A. Tamilvanan, K. Balamurugan, K. Ponappa, and B. Madhan Kumar, “Using Response Surface Methodology in Synthesis of Ultrafine Copper Nanoparticles by Electrolysis,” Int. J. Nanosci., vol. 15, no. 01n02, p. 1650001, 2016.
[۲۱] R. K. Nekouei, F. Rashchi, and A. A. Amadeh, “Using design of experiments in synthesis of ultra-fine copper particles by electrolysis,” Powder Technol., vol. 237, pp. 165–۱۷۱, ۲۰۱۳٫
[۲۲] T. Theivasanthi and M. Alagar, “Nano sized copper particles by electrolytic synthesis and characterizations,” Int. J. Phys. Sci., vol. 6, no. 15, pp. 3662–۳۶۷۱, ۲۰۱۱٫
[۲۳] M. Raja, “Production of copper nanoparticles by electrochemical process,” Powder Metall. Met. Ceram., vol. 47, no. 7–۸, pp. 402–۴۰۵, ۲۰۰۸٫
[۲۴] H. Wang, W. Xia, and B. Ren, “Power Consumption Model for Electrolytic Preparation of Copper Powders Using Response Surface Methodology.”
[۲۵] H. Hashemipour, M. E. Zadeh, and R. Pourakbari, “Investigation on synthesis and size control of copper nanoparticle via electrochemical and chemical reduction method,” vol. 6, no. 18, pp. 4331–۴۳۳۶, ۲۰۱۱٫
[۲۶] K. S. Tan and K. Y. Cheong, “Advances of Ag, Cu, and Ag-Cu alloy nanoparticles synthesized via chemical reduction route,” J. Nanoparticle Res., vol. 15, no. 4, 2013.
[۲۷] W. Yu, H. Xie, L. Chen, Y. Li, and C. Zhang, “Synthesis and characterization of monodispersed copper colloids in polar solvents,” Nanoscale Res. Lett., vol. 4, no. 5, pp. 465–۴۷۰, ۲۰۰۹٫
[۲۸] Q. L. Zhang, Z. M. Yang, B. J. Ding, X. Z. Lan, and Y. J. Guo, “Preparation of copper nanoparticles by chemical reduction method using potassium borohydride,” Trans. Nonferrous Met. Soc. China (English Ed., vol. 20, no. SUPPL.1, pp. 2–۶, ۲۰۱۰٫
[۲۹] S. V. Saikova, S. A. Vorob’ev, R. B. Nikolaeva, and Y. L. Mikhlin, “Conditions for the formation of copper nanoparticles by reduction of copper(II) ions with hydrazine hydrate solutions,” Russ. J. Gen. Chem., vol. 80, no. 6, pp. 1122–۱۱۲۷, ۲۰۱۰٫